Speaking of lithium-ion batteries, we are no strangers, whether it is a mobile phone or a laptop, and ultimately, the presence of lithium-ion batteries. With the continuous development of electronic technology, our requirements for lithium-ion batteries are also increasing. We need lithium. Ion batteries have higher energy density and better safety, which poses more challenges for the development of lithium-ion batteries. The key to improving the energy density of lithium-ion batteries is to increase the capacity of the positive and negative active materials. The traditional capacity of graphite materials is about 300mAh/g, and LCO material capacity is about 140mAh/g, which greatly limits the lithium-ion battery. Increased energy density. In recent years, with the development of materials technology, positive and negative electrode materials with high capacity are also emerging. For example, negative electrode silicon materials, positive electrode ternary materials, and lithium-rich materials far exceed the conventional lithium-ion battery electrodes. The materials have greatly promoted the increase of energy density of lithium-ion batteries.
Due to its advantages of high capacity and rich resource reserves, silicon anode materials have achieved great development in recent years and are currently the most mature high-capacity anode materials. The silicon anode materials are mainly divided into two major categories: crystalline silicon and oxidized silicon materials. Although the silicon materials have high capacity, there are still many problems, such as large expansion of crystalline silicon, poor cycle performance, and low efficiency of the first coulomb of oxidized silicon. To this end, people are constantly developing various new types of high-capacity negative electrode materials, such as N-doped graphite materials, lithium transition metal transition materials, and metal lithium negative materials, which are currently playing a positive role. Recently, the Chinese Academy of Sciences Changchun should Dongming Yin et al. designed a novel adhesive-free CuO nanorod electrode obtained by directly growing porous CuO nanorod arrays on Cu foils. This method avoids the use of adhesives and conductive agents. This allows better contact between the CuO active material and the Cu foil. The specific capacity of the electrode can reach more than 670mAh/g (100mA/g) or more, but it has good rate performance and cycle performance (under a current density of 100mA/g, the remaining capacity of the cycle can reach 671mAh/g).
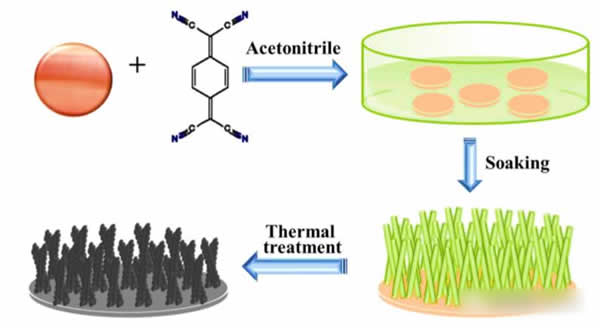
The theoretical capacity of CuO material reaches 670mAh/g, and it has the advantage of abundant resource reserves. However, CuO also faces the problem of excessive volume expansion during charge and discharge processes. Nanonization is a common solution to the problem of large expansion of materials. To overcome the problem of large expansion of the CuO electrode, Dongming Yin used the MOFs method to directly grow CuO porous nanorods on the copper foil. The specific preparation method of the electrode is as shown in the above figure. First, the copper foil is immersed to 7; 7,8,8-tetracyanoquinoline methane TCNQ and TCNQ react with Cu foil to form Cu-TCNQ. After heat treatment at 250 °C, Cu-TCNQ will be converted into CuO nanorods.
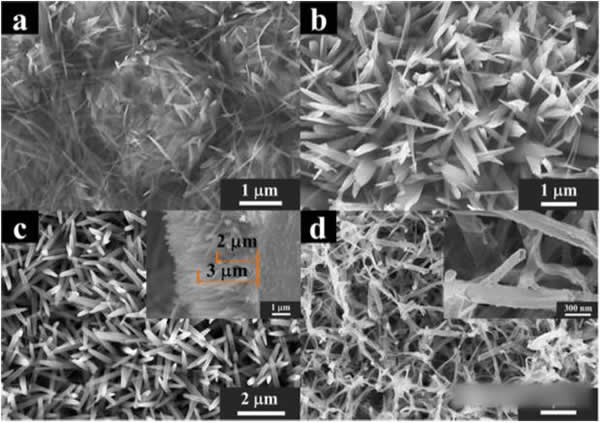
The morphologies of the Cu-TCNQ precursor obtained at different times in the TCNQ treatment and the treatment at 250°C to obtain CuO are shown in the figure below. From the figure, we can see that the number of precursor nanorods gradually increases with the processing time. With the increase, after treatment at 250 °C, CuO inherits the morphology of the precursors and connects with each other to form a porous structure.
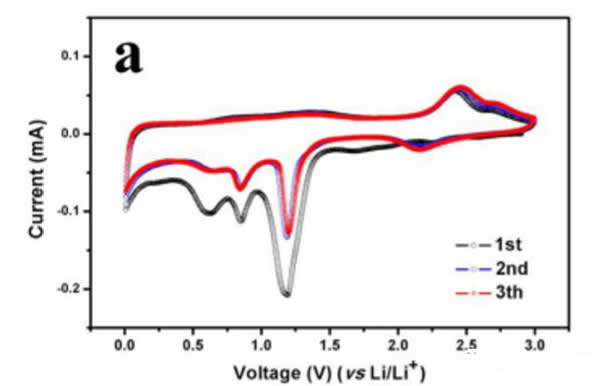
Dongming Yin performed an electrochemical test on the CuO negative electrode prepared above. The following figure shows the results of the cyclic voltammetry test. From the figure, it can be seen that a weak current peak appears around 1.75V in the first reduction process, at 1.19. A strong current peak appears in V. There are two moderate current peaks at 0.85V and 0.62V, respectively. The electrochemical reactions that occur during this process are shown below.
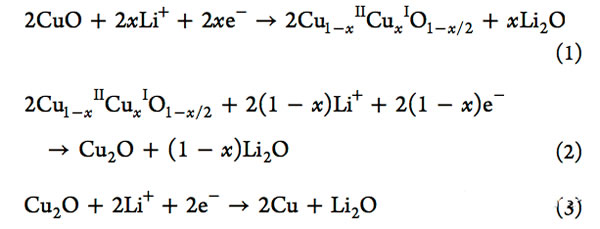
The capacity test showed that the capacity of the electrode reached 670mAh/g or more, and had excellent cycle and rate performance. The cycle capacity was 150 cycles at a current density of 100mA/g. The electrode capacity can reach 671mAh/g, and the rate test also shows that The electrode has excellent electrochemical performance, and at current densities of 100, 200, 500 and 1000 mAh/g, the electrode capacity can reach 730, 554, 434 and 367 mAh/g, respectively, even at a current of 2000 mA/g. At a density, the capacity of the material can still reach 300 mAh/g.
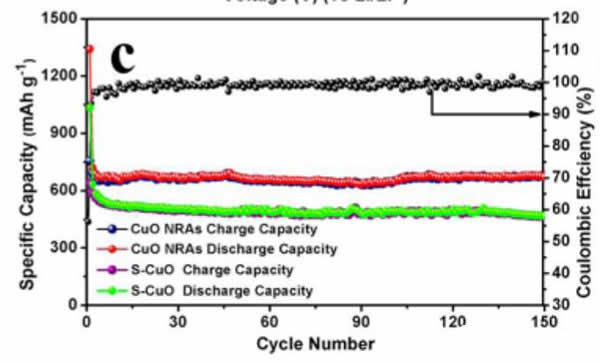
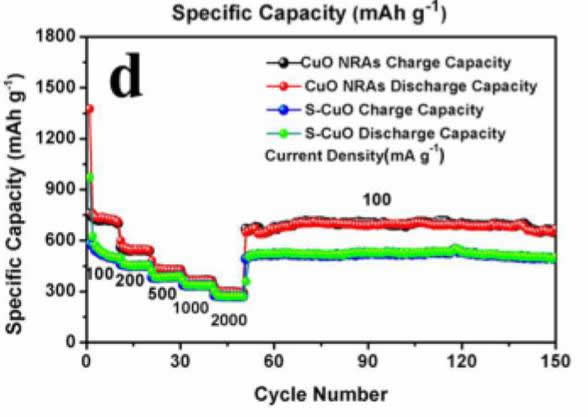
The biggest problem with this electrode is that it has low efficiency for the first time. When the electrode is first charged, its charge capacity can reach 1341 mAh/g, far higher than its theoretical capacity of 670 mAh/g, but the first efficiency is only 58%, resulting in this There are two main reasons for a phenomenon: 1) Li2O generated during the reaction is not reactive; 2) The electrolyte decomposes on the surface of CuO, and the larger specific surface area of ​​the nanomaterial increases the amount of decomposition of the electrolyte, resulting in the CuO electrode. The first time efficiency is low. Therefore, this material application also needs to be used in conjunction with lithium supplementation technology.
Dongming Yin et al. developed a method for preparing nanorod electrode material directly on metal foil by using metal organic compound MOFs method. The process is simple and suitable for large-scale production, and can be extended to electrodes of other materials, such as directly growing SiOx nanorods on copper foil. And so on, using the advantages of nanomaterials to overcome the volume expansion of the material in the charge and discharge process, so this method has a very good application prospects.
Steel Measuring Tape,Aluminum Level,Fiberglass Tape Measure,Digital Depth Gauges
SHAOXING SINO IMPORT & EXPORT CO.,LTD , https://www.sxsmarto.com