Figure 2 Schematic diagram of cutting
1. Process cylinder 2. Workpiece 3. Cutting knife
Although the process scheme can meet the process requirements of the parts, the material cost of the part blanks and the labor cost of the welding are increased, which is not economical. Based on the idea of ​​cost saving and product quality improvement, we have carefully designed the turning tooling of thin-walled taper parts. When turning a thin-walled taper-type part turning tooling tool, the part process is divided into the following two processes.
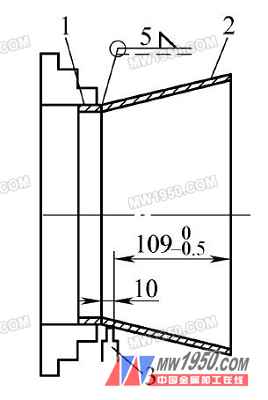
Car cone hole process
(1) Inside the cone hole tooling structure The tooling consists of two parts, a positioning sleeve 2 and a clamping nut 4, as shown in Fig. 3.
Previous page next page
The COVID-19 Antigen Saliva Test kit is a lateral flow immunoassay intended for the preliminary screening and qualitative detection of nucleocapsid protein antigen from severe acuterespiratorysyndrome coronavirus SARS-CoV-2which causesthe Coronavirusdisease(COVID-19),in direct saliva sample from individuals suspected of coVD-19 by their healthcare provider within the first seven days of symptom onset.The COVID-19 Antigen Saliva Test kit is for professional use only and is intended to be used as an aid in the diagnosis of SARS CoV-2 infection.
SARS-CoV-2is anenvelopedsingle-stranded RNAvirus ofthe Baenus COVD-19 is an acute respiratory infectious disease. People are generally susceptible.Currently. the patients infected bytheSARS-CoV2are the main source ofinfection:asvmptomatic infected peope car also be an infectious source Based on thecurrentepidemiologica investigation, the incubation period is 1to 14 daysmostly 3to 7 days The main manifestations include feverfatigue and drycough.Nasa congestion, runny nosesore throatmyalgia and diarhea are found in a few cases.
Results are for the identification of SARS-CoV-2 nuceocapsid protein antigen. The SARS-CoV-2 antigen is generally detectable in upper respiratory specimens during the acute phase ofinfectionPositive results indicate the presence of SARS-CoV-2viralantigenshowever cinicai corelation with patient history and other diaanostic information is key to determine status of infection.Positive results don't preclude bacterialinfection or co-infection with other yiruses.eaatveresuts To patients with symptom onset for more than seven days should be treated as presumptive and confirmation with a molecular assayand patient management may be performed according to local regulations as needed.
saliva antigen rapid test kit,antigen test saliva,antigen saliva test kit,rapid antigen saliva
Yong Yue Medical Technology(Kunshan) Co.,Ltd , https://www.yonyuemedicalcare.com